PREVAIL Epilepsy Trial
Qudexy® XR is an extended-release topiramate formulation backed by PREVAIL phase 3 data in patients with epilepsy.
Trial Design
The efficacy and safety of Qudexy® XR as adjunctive treatment for adults with partial-onset seizures were evaluated in PREVAIL.1,2
- Randomized, double-blind, parallel-group, placebo-controlled trial
- Enrolled patients with a history of partial-onset seizures (POS) on a stable dose of 1 to 3 AEDs
- Seizure frequency during 8-week baseline: ≥8 POS, with or without secondary generalization, and ≤21 consecutive seizure-free days
- 249 patients were randomly assigned to Qudexy® XR once daily or placebo in addition to their AEDs
- Most common concomitant AEDs included carbamazepine, valproic acid, lamotrigine and/or levetiracetam*
- Treatment consisted of an initial 3-week titration period followed by an 8-week maintenance period at 200 mg once daily of Qudexy® XR or placebo
*See Table 10 in the Qudexy® XR Prescribing Information for a summary of AED interactions with topiramate.
PREVAIL Titration Schedule
- Initial Dose
- Titration
- Maintenance Dose
- 50 mg once daily
- Increased dose weekly by increments of
50 mg for 3 weeks - 200 mg once daily
Open-Label Extension of PREVAIL
96.8% of patients who completed PREVAIL continued into the open-label extension study.1,2
Patients who completed the PREVAIL treatment phase were eligible to enroll in the 1-year open-label extension.
- Patients underwent a 3-week blinded-conversion phase followed by a 52-week open-label treatment phase
- After 11 weeks of the open-label extension:
- Qudexy® XR dose could be changed in 50 mg/week increments (maximum dose: 400 mg daily)
- Concomitant AEDs could be added, removed or dose-adjusted as long as patients remained on 1 to 3 concomitant AEDs
Efficacy
Qudexy® XR demonstrated a nearly 40% reduction from baseline in refractory partial-onset seizures (POS) with only a 200 mg maintenance dose.1,2
PREVAIL Primary Endpoint During 11-Week Treatment Period
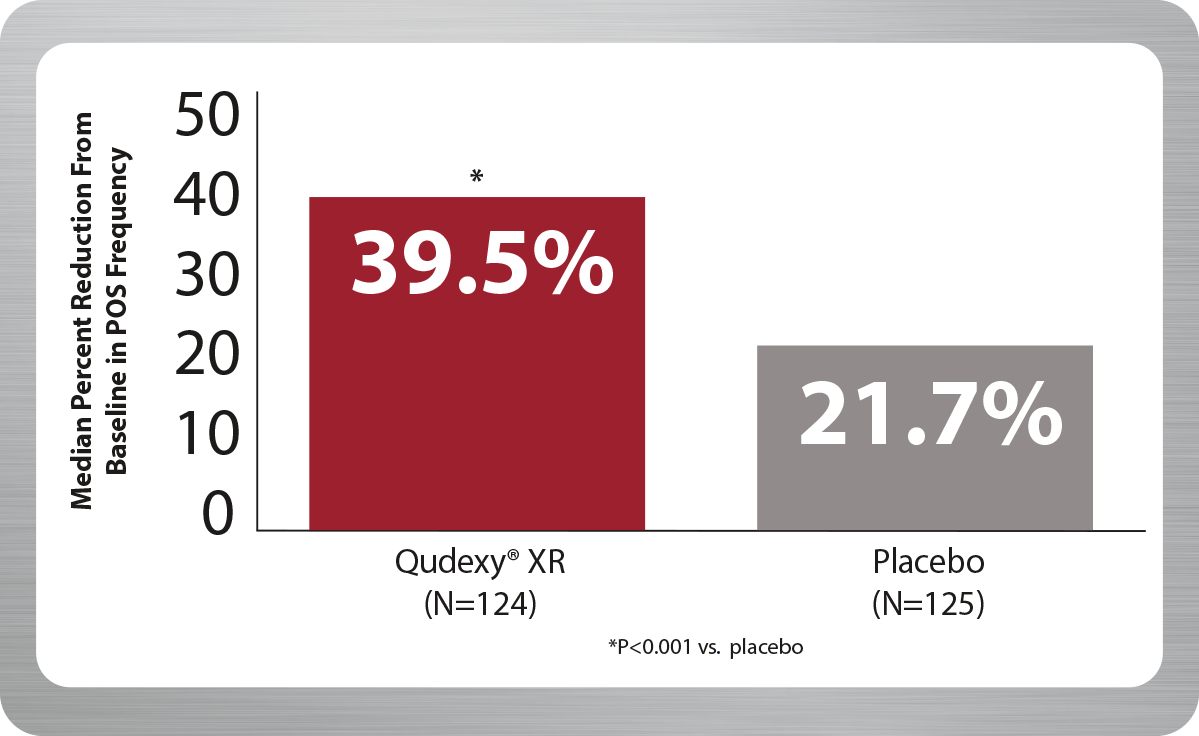
Although this controlled clinical trial was performed to assess the efficacy and safety of Qudexy® XR in patients, the basis for approval of Qudexy® XR included the studies using an immediate-release topiramate formulation and the demonstration of the pharmacokinetic equivalence of Qudexy® XR to immediate-release topiramate through the analysis of concentrations and cumulative AUCs at multiple time points.
POS=partial-onset seizure; AUC=area under the curve.
Qudexy® XR is an extended-release topiramate formulation with contemporary topiramate PREVAIL efficacy data when used with other AEDs, including carbamazepine, valproic acid, lamotrigine and levetiracetam.†2
† See Table 10 in the Qudexy® XR Prescribing Information for a summary of AED interactions with topiramate.
Favorable Safety and Tolerability Profile
Most treatment-emergent adverse events (TEAEs) reported were mild to moderate in intensity.1,2
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the Qudexy® XR trial, a dose of 200 mg/day was administered to a limited number of patients; therefore, these results cannot be directly compared to immediate-release topiramate experience.
Incidence (≥2%) of Treatment-Emergent Adverse Reactions in the PREVAIL Trial
| Body System/Adverse Reaction | Qudexy® XR (N=124) | Placebo (N=125) |
General disorders
| Fatigue | 6% | 5% |
| Asthenia | 2% | 1% |
| Irritability | 2% | 1% |
Nervous system disorders
| Somnolence | 12% | 2% |
| Dizziness | 7% | 6% |
| Paresthesia | 7% | 2% |
| Aphasia | 2% | 0% |
| Dysarthria | 2% | 1% |
| Memory impairment | 2% | 1% |
Psychiatric disorder
| Psychomotor retardation | 2% | 0% |
Cardiovascular disorders, general
| Hypertension | 3% | 1% |
Metabolic and nutritional disorders
| Weight decrease | 7% | 0% |
| Decreased appetite | 4% | 2% |
| Anorexia | 2% | 1% |
8.9% of patients who received Qudexy® XR and 4.0% who received placebo discontinued as a result of TEAEs.
References:
1. Qudexy® XR [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, Inc.; February 2019.
2. Data on file. Maple Grove, MN: Upsher-Smith Laboratories, Inc.; 2019.

 Rotate for Important Safety Information
Rotate for Important Safety Information